What is the carbon footprint of the different steel production technologies?
Steel is a fundamental key material in the global economy and modern civilization, serving as the backbone of infrastructure, energy, industry, manufacturing, safety and transportation systems. With an annual global production of nearly 2 billion metric tons, steel is an essential component in a wide range of industries. Its versatility, formability, damage-tolerance, durability, and strength make it an ideal material for construction, manufacturing, and engineering applications.
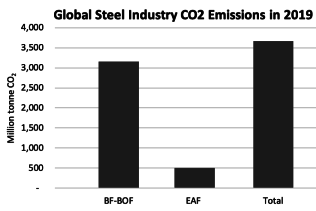
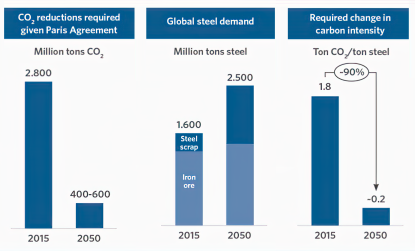
In 2022, the steel industry was responsible for emitting approximately 2.6-2.7 billion metric tons of CO2eq, accounting for roughly 7-8% of all global emissions. of this greenhouse gas. This places steel among the top three highest emitting materials, alongside aluminum and cement. On average, the production of one ton of crude steel results in the emission of two tons of CO2eq in the top 15 steel-producing countries. Addressing the issue of emissions from steel production is not only a geopolitical and geographical challenge, as the steel industry is strategically important and many countries prioritize regional production and downstream manufacturing over imports, but it is also a huge challenge for basic research and advanced engineering.
What causes steel's enormous CO2eq emissions?
Several process steps contribute to the high greenhouse gas emissions:
1. Mining, extraction and benefication
The extraction of iron ore is relatively carbon efficient, with an average emission of 0.25 tCOe per ton of ore extracted. However, the carbon footprint of iron ore extraction can vary significantly depending on the quality of the ore deposits and the geology of the region. Some iron ores require more processing after mining, which can increase their carbon footprint.
2. Reduction from oxides into iron (or pig-iron Fe-C (eutectic))
To produce steel from iron ore, it first needs to be reduced into iron. There are several main process methods used for this:
Blast Furnace (BF) route:
In a blast furnace, iron oxide is reduced into molten iron (pig iron) and carbon dioxide, by adding carbon (as coke) and oxygen from a hot air blast, following the highly simplified net balance redox equation:
2C + O2 → 2CO
Fe2O3 + 3CO → 2Fe + 3CO2
This is followed by melting the Fe-C near eutectic prig-iron from the blast furnace with a Basic Oxygen Furnace (BOF), called converter. Used together, BF-BOF form the modernized version of the Bessemer process.
The blast furnace production of pig iron is a particularly carbon-intensive process due to the use of coke as a reducing agent. The coke is produced by heating coal in the absence of air, which drives off volatile compounds and leaves behind a porous, carbon-rich material. This coke is then used in the blast furnace to reduce iron ore to pig iron, a process that involves the reaction of carbon with oxygen to produce carbon dioxide (CO2).
In addition to the CO2 emissions produced by the reduction reaction itself, there are also significant emissions associated with the production of coke. The coking process involves heating coal to high temperatures, which releases large amounts of CO2 and other greenhouse gases. Furthermore, the mining and transportation of coal also contribute to the carbon footprint of blast furnace steelmaking.
Overall, the use of coke as a reducing agent in blast furnace steelmaking is a major source of CO2 emissions. Efforts are being made to develop alternative methods of producing pig iron that are less carbon-intensive, such as direct reduced iron (DRI) using hydrogen or natural gas.
Direct reduced iron (DRI) route:
The direct reduction of iron ore does not require the use of a blast furnace. Instead, it utilizes a mixture of hot gases, often hydrogen and carbon monoxide, to reduce the ore. Natural gas is commonly used as a source of these gases where it is readily available and inexpensive. The resulting product, known as sponge iron, can then be melted into steel using electric arc furnaces (EAFs). This process is generally considered to be lower in carbon emissions than traditional blast furnace methods, as it primarily uses natural gas or hydrogen alternatives rather than coal. However, this is not always the case
Overall, the direct reduction technology is a less CO2 intense production route for steel than the blast furnace route because it relies on the use of reducing gases, such as hydrogen and carbon monoxide, rather than coke to reduce iron ore to iron. Coke is produced by heating coal in the absence of air, which releases large amounts of CO2 and other greenhouse gases. In contrast, the reducing gases used in direct reduction can be produced from natural gas or other sources that have a lower carbon footprint.
In addition to the lower emissions associated with the production of reducing gases, direct reduction also has the potential to be powered by renewable energy sources. For example, hydrogen can be produced through electrolysis using electricity generated from renewable sources such as wind or solar power. This further reduces the carbon footprint of direct reduction compared to blast furnace steelmaking.
Overall, direct reduction offers a more sustainable alternative to traditional blast furnace methods for producing steel. By using reducing gases rather than coke and by utilizing renewable energy sources, direct reduction has the potential to significantly reduce CO2 emissions from steelmaking.
3. Smelting
Smelting iron into steel is mainly done in a Basic Oxygen Furnace (BOF) or Electric Arc Furnace (EAF):
Basic Oxygen Furnace (BOF)
To make steel from pig iron, impurities such as carbon and silicon need to be removed. In a BOF, this is done mostly by oxidation, but also by interaction with various alloying elements (elements added to increase corrosion resistance and strength).
Oxygen is blown as gas into the furnace, unlike in the Bessemer process, where oxygen-carrying substances are added into the heat to burn excess carbon away.
Electric Arc Furnaces (EAF)
An electric arc furnace (EAF) is a type of metallurgical furnace that uses an electric arc to heat and melt materials, primarily metal ore or scrap metal, in the production of steel. These furnaces range in capacity from a few tons to as many as 400 tons, and a steel melting shop can have a single furnace or up to three or four.
The EAF operates by applying an AC current to a steel scrap charge through graphite electrodes. This creates an electric arc that generates intense heat, melting the scrap and converting it into liquid steel. The process requires a large amount of electricity and can cause disturbances in power grids due to the high energy demand in a short period of time.
In addition to melting scrap steel, EAFs can also be used to convert direct reduced iron (DRI) into liquid steel, achieving the same quality as can be achieved in an integrated steel plant. The EAF also produces valuable slag as a by-product, similar to the blast furnace.
The EAF operates as a batch melting process, producing batches of liquid steel. The operating cycle, known as the tap-to-tap cycle or heat, consists of several components: charging the furnace with scrap or DRI, melting the charge, refining the melt, deslagging, tapping the liquid steel, and preparing the furnace for the next heat. Modern EAFs can achieve tap-to-tap times of less than 60 minutes, with some twin-shell furnace operations achieving times as low as 35-40 minutes.
Overall, the electric arc furnace is a versatile and efficient method for producing steel from scrap or DRI. Its ability to produce high-quality steel using electricity rather than burning fossil fuels makes it an attractive option for reducing CO2 emissions from steelmaking.
In sumary EAFs use large amounts of high voltage electricity to heat iron to over 1,000°C. This drives off impurities and allows steel to be reformed.
The main sources of iron used in an EAF are scrap steel and DRI (often both).
This process is less carbon-intense than BOF, mainly due to the use of electricity to produce heat. EAFs are nearly always lower carbon than BF-BOFs, although this depends on the electricity mix of the country.
Carbon balance
The DRI+EAF process is lower carbon than BF-BOF in almost all cases. Per tonne of crude steel:
DRI+EAF emits an average of 1.2 tCOe
BF-BOF emits an average of 2.2 tCOe
The World Steel Association (Worldsteel) suggests the use of the so called ‘system expansion’ method for the life-cycle assessment for steels. This is a rather comprehensive assessment method and follows the ISO 14040 series of environmental standards. It also gives credit for the net CO2 that is saved when a product is reused or recycled, which is often the case for steels.
Accordign to this estimatation method, the conventional blast furnace and BOF route for primary production uses globally about 14% scrap (and the rest pig iron), with emissions of 1.987 tonnes of CO2/tonne of steel. The secondary path, where the steel is produced in EAFs from scrap,uses as total input 105% scrap steel, with emissions of 0.357 tonnes CO2/tonne. The ‘system expansion’ calculation method considers the full product life-cycle from ‘cradle to grave’.
Strategies to reduce the CO2 emissions from steelmaking
Several methods are currently being developed by scientists to reduce CO2 emissions from steelmaking. One promising approach is to transition away from traditional blast furnace methods, which rely on using coal as reductant precursor, towards more sustainable alternatives such as electric arc furnaces (EAFs) and direct reduced iron (DRI) using hydrogen or natural gas.
Carbon capture and storage (CCS) is another technique that is being proposed by some companies to reduce carbon dioxide emissions in the steel industry, however this method has neither enough space to take on all the global CO2 emissions from steelmaking not is it truly sustainable. It involves capturing the CO2 emissions produced during steelmaking and either utilizing them in other industrial processes (such as green fuel production, which is indeed considered by scientists to be a very useful approach) or storing them underground to prevent their release into the atmosphere (which is considered not to be sustainable by scientists).
In addition to these transient technological solutions, there are also efforts being made to improve the efficiency of existing steelmaking processes. For example, methods such as coke dry quenching, optimizing pellet ratios, and using top gas recovery turbines can help to reduce conventional primary route carbon emissions. Replacing coke with natural gas or injecting hydrogen or ammonia into the blast furnace can also significantly cut CO2 emissions in primary steelmaking.
Overall, reducing CO2 emissions from steelmaking is a complex challenge that will require a combination of basic research into novel sustainmable metallurgical production methods (such as hydrogen-based plasma reduction smelting or H-DRI), technological innovation, improved efficiency, and changes in industry practices. However, with growing pressure to decarbonize and increasing demand for sustainable steel products, there are significant research opportunities to develop new ideas.