Simulation of iron oxide reduction with hydrogen using a phase field model
The reduction of iron ore with coke-based carbon is the largest single source of greenhouse gas emissions and global warming. This has triggered research activities to replace the coke-based blast furnace reduction by hydrogen-based direct reduction (HyDR).
Iron oxide reduction with hydrogen is investigated experimentally and theoretically. The HyDR process includes multiple types of chemical reactions, solid state and defect-mediated diffusion (of oxygen and hydrogen species), several phase transformations, as well as massive volume shrinkage and mechanical stress buildup.
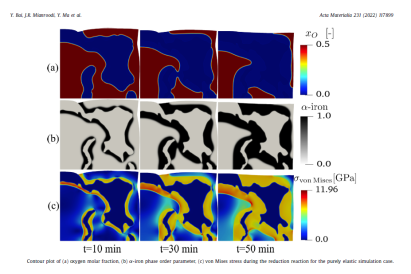
However, studies focusing on the chemo-mechanical interplay during the reduction reaction influenced by microstructure are sparse. In this work, a chemo-mechanically coupled phase-field (PF) model has been developed to explore the in- terplay between phase transformation, chemical reaction, species diffusion, large elasto-plastic deforma- tion and microstructure evolution. Energetic constitutive relations of the model are based on the system free energy which is calibrated with the help of a thermodynamic database. The model has been first ap- plied to the classical core-shell (wüstite-iron) structure. Simulations show that the phase transformation from wüstite to α-iron can result in high stresses and rapidly decelerating reaction kinetics. Mechanical stresses create elastic energy in the system, an effect which can negatively influence the phase transfor- mations, thus causing slow reaction kinetics and low metallization. However, if the elastic stress becomes comparatively high, it can shift the shape of the free energy from a double-well to a single-well case, speed up the transformation and result in a higher reduction degree compared to the low-stress double- well case. The phase field model has been applied to simulate an experimentally characterized iron oxide specimen with its complex microstructure. The observed microstructure evolution during reduction is well predicted by the model. The simulation results also show that isolated pores in the microstructure are filled with water vapor during reduction, which can influence the local reaction atmosphere and dynamics.
In our paper "Chemo-mechanical phase-field modeling of iron oxide reduction with hydrogen" we focused on developing a model to understand the hydrogen-based direct reduction (HyDRI) of iron oxide. This process is significant as it offers a more environmentally friendly alternative to traditional iron ore reduction methods, which are major contributors to greenhouse gas emissions. The model aims to explore the complex interplay between phase transformations, chemical reactions, species diffusion, and large elasto-plastic deformations in the context of microstructure evolution during iron oxide reduction with hydrogen.
The study addresses a critical gap in existing research by focusing on the chemo-mechanical interactions during the reduction process, influenced by the microstructure. It highlights that the HyDRI process encompasses multiple chemical reactions, solid-state and defect-mediated diffusion of oxygen and hydrogen, phase transformations, as well as considerable volume shrinkage and mechanical stress buildup. The model developed in this work incorporates energetic constitutive relations based on system free energy, calibrated using a thermodynamic database. It has been applied to classical core-shell (wüstite-iron) structures, demonstrating that phase transformation from wüstite to α-iron can cause high stresses and decelerate reaction kinetics. These mechanical stresses, by creating elastic energy, can either negatively influence phase transformations, resulting in slow kinetics and low metallization, or, if sufficiently high, can accelerate the phase transformation, enhancing reduction efficiency.
The paper also reviews previous research on the physical and chemical processes involved in HyDRI, including kinetics, diffusion, phase transformation, mechanical deformation, and pore formation, and their impact on the effectiveness of HyDRI. Studies have shown that various factors, such as counter-diffusion of gases and structural variations in iron oxide, significantly influence reduction kinetics. Moreover, the microstructure of iron ore and its evolution during reduction are critical aspects of these investigations, determined by several phase transformations during the HyDRI process
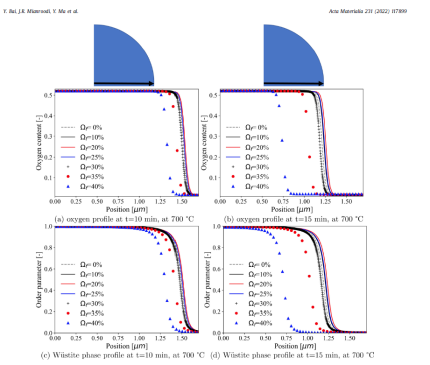
The paper introduced, tested, and applied a chemo-mechanically cou- pled phase-field (PF) model to study the iron oxide direct reduc- tion with gaseous hydrogen. The constitutive laws for the diffu- sion of oxygen, the phase transformation from wüstite to α-iron, as well as the elasto-plastic deformation have been derived from the system free energy. The model makes use of an existing ther- modynamic database for the oxygen-dependent free energies of wüstite and α-iron. In particular, the thermodynamic database for the oxygen-dependent free energies of wüstite and α-iron has been incorporated within this PF model. We have first benchmarked our model for an oxygen-dependent free energy scenario in a rectangular domain. Simulation results show that the predicted phase fractions in the
Acta Mater 2022 Chemo-mechanical phase-f[...]
PDF-Dokument [4.6 MB]