Welding: Microstructures, intermetallics, mechanical properties
Dissimilar material welding: Joining steel to aluminum
Aluminum compounds can be joined to most other materials quite readily by adhesive bonding or mechanical fastening.
In contrast, special care and specific metallurgical techniques must be applied if Aluminum compounds are to be arc welded to other alloys such as steel. In such cases very brittle and hence
undesired intermetallic compounds can be formed when metals such as steel, copper, magnesium or titanium are directly arc welded to aluminum. To avoid these brittle compounds, some special techniques
have been developed to isolate the other metal from the molten aluminum during the arc welding process. The two most common methods of facilitating arc welding of aluminum to steel are bimetallic
transition inserts and coating the dissimilar material prior to welding.
Atom probe tomography of intermetallic phases and interfaces formed in dissimilar joining between Al alloys and steel
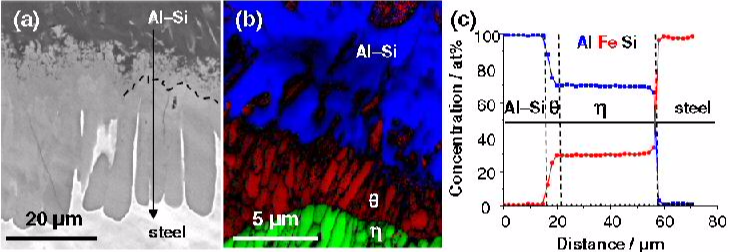
While Si additions to Al are widely used to reduce the thickness of the brittle intermetallic seam formed at the interface during joining of Al alloys to steel, the underlying mechanisms are not clarified yet. The here presented approach for the site specific atom probe tomography analysis revealed Si enrichments at grain and phase boundaries between the θ (Fe4Al13) and η (Fe2Al5) phase, up to about ten times that of the concentration in Al. The increase in Si concentration could play an important role for the growth kinetics of the intermetallic phases formed for example in hot-dip aluminizing of steel.
Materials Characterization 120 (2016) 268–272
Atom probe tomography intermetallic phas[...]
PDF-Dokument [1.4 MB]
Laser beam welding of dual-phase steel
Autogeneous laser beam welding is an efficient process to join ferritic-martensitic dual-phase steels without large dimensional distortions. Localized softening may occur in the heat affected zone, particularly in DP1000 steel with a high martensite volume fraction. DP1000 steel was welded in bead-on-plate configuration varying the welding power between 0.4 and 2.0 kW and the welding speed between 20 and 150 mm/s. Light optical microscopy, scanning electron microscopy, and X-ray diffraction were used to perform the microstructural characterization of the welded joints. High-quality laser beam welds of thin sheets of DP1000 steel can be produced using appropriate welding parameters. The optimal welding condition was a nominal laser power of 2.0 kW and a welding speed of 150 mm/s. This condition minimizes the amount of softening of prior martensite and yields a narrow heat affected zone and a small volume fraction of retained austenite in the weld.
Journal of Materials Processing Tech. 252 (2018) 498–510
Dual Phase Steel Laser Beam welding micr[...]
PDF-Dokument [2.7 MB]
Dissimilar joining of steel to aluminium alloys: the Kirkendall effect
Dissimilar joining of iron (Fe) to aluminium (Al) alloys is of eminent technical interest, as it allows the use of these two essential engineering materials in the same design. Thus, the high strength, good creep resistance, and formability of steels may be combined with the low density, high thermal conductivity and
good corrosion resistance of Al alloys in one hybrid part. Applications for such dissimilar joints between Fe and Al alloys are ubiquitous and range from an improved
strength-to-weight ratio in transportation systems to high temperature corrosion protection in the chemical industry or enhanced cooling efficiency
for example in cryogenic technology. Joining of Fe and Al alloys is impeded by the differences in their mechanical properties and melting temperatures and because of the formation of
brittle intermetallic phases.
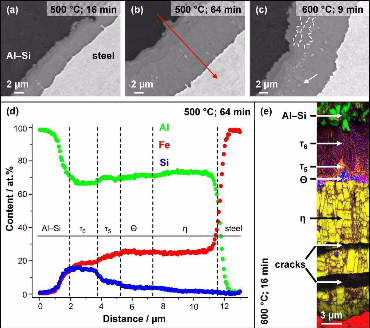
In this work the formation of intermetallic reaction layers and their influence on mechanical properties was investigated in friction stir welded joints between a low C steel and both pure Al (99.5 wt.%) and Al–5 wt.% Si. Characterisation of the steel/Al interface, tensile tests and fractography analysis were performed on
samples in the as-welded state and after annealing in the range of 200–600°C for 9–64 min. Annealing was performed to obtain reaction layers of distinct thickness and composition. For
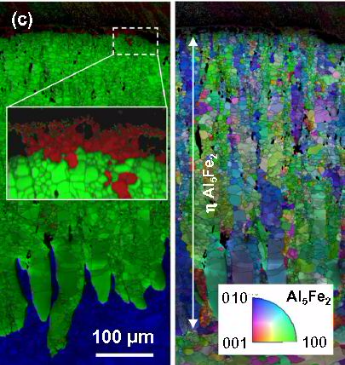
both Al alloys, the reaction layers grew with parabolic kinetics with the h phase (Al5Fe2) as the dominant component after
annealing at 450 ◦C and above. In joints with pure Al, the tensile strength is governed by the formation of Kirkendall-porosity at the reaction layer/Al interface. The tensile strength of
joints with Al–5 wt.% Si is controlled by the thickness of the h phase (Al5Fe2) layer. The pre-deformation of the base materials, induced by the friction stir welding procedure, was
found to have a pronounced effect on the composition and growth kinetics of the reaction layers.
H. Springer, A. Kostka, J.F. dos Santos, D. Raabe
Materials Science and Engineering A 528 (2011) 4630-4642
Influence of intermetallic phases and Kirkendall porosity on the mechanical properties of joints between steel and aluminium alloys
Mater Sc Engin A 528 (2011) 4630 Kirkend[...]
PDF-Dokument [1.2 MB]
What is formation mechanisms and role of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys ? Basic study on dissimilar steel to aluminum welding
Iron (Fe) and aluminum (Al) alloys rank among the most important engineering materials because they provide good properties at low material cost in many applications [1]. Fe is the primary
constituent of steels, which are versatile in structural applications where good creep resistance, formability or high strength are required [2,3]; Al alloys can provide excellent value in
applications where low density and/or good corrosion resistance are desired [4,5]. Dissimilar joints between Fe and Al alloys are of significant importance due to the ubiquity of these materials in
engineering applications. The ability to form dissimilar joints between Fe and Al alloys is attractive because such bonds offer design engineers flexibility in many situations. While there is
the obvious case of improving the strength-to-weight ratio in construction and transportation systems [6,7], there also exist many other situations in which a good bond between Fe and Al alloys is
highly desirable, such that the differing material properties of Al and Fe/steel may be exploited. For example, such joints may simultaneously provide high thermal conductivity and high-temperature
corrosion resistance in household applications [8] as well as in the chemical industry [9]. In addition, they are used in the smelting production of Al [10]. While the specific properties that
define a “good” bond vary between applications, it is unisersally acknowledged that joining of Fe and Al alloys is hindered by the large difference in melting temperatures, the difference in
mechanical properties, and because of the formation of brittle intermetallic phases. Here, the formation of intermetallic reaction layers was investigated for interdiffusion between a low-carbon
steel and commercially pure aluminum (99.99%) and between a low-carbon steel and an aluminum–silicon alloy (Al–5 wt.% Si). Solid/solid, solid/semi-solid and solid/liquid diffusion couples were
produced at both 600 and 675°C. The total width of the reaction layer is governed mainly by the parabolic diffusion-controlled growth of the g phase (Al5Fe2), which exhibits orientation-dependent
growth kinetics. The addition of
Si to Al, which is known to decelerate reaction layer growth in interdiffusion experiments with Al melts, was found to accelerate the reaction layer growth in solid/semi-solid interdiffusion
experiments. This phenomenon is discussed in light of previous atomistic explanations and the apparent activation energy calculated for the growth of the g phase (Al5Fe2). See also details in
:
Acta Materialia 59 (2011) 1586-1600 H. Springer, A. Kostka, E.J. Payton, D. Raabe, A. Kaysser-Pyzalla, G. Eggeler On the formation and growth of intermetallic phases during interdiffusion between
low-carbon steel and aluminum alloys.
Acta Materialia 59 (2011) 1586-1600
H. Springer, A. Kostka, E.J. Payton, D. Raabe, A. Kaysser-Pyzalla, G. Eggeler
On the formation and growth of intermetallic phases during interdiffusion between low-carbon steel and aluminum alloys
Acta Materialia 59 (2011) 1586 Steel Alu[...]
PDF-Dokument [1.3 MB]
Agnieszka Szczepaniak, Jianfeng Fan, Aleksander Kostka and Dierk Raabe
ADVANCED ENGINEERING MATERIALS 2012, 14, No. 7, page 464
On the Correlation Between Thermal Cycle and Formation of Intermetallic Phases at the Interface of Laser-Welded Aluminum-Steel Overlap Joints
ADVANCED ENGINEERING MATERIALS 2012, 14,[...]
PDF-Dokument [519.7 KB]
A laser beam welding process via heat conduction was applied to join DC01 steel with aluminum (Al) in overlap configuration without filler wire. The effect of the applied laser power (1.7,
1.8, 2.1, and 2.4 kW) on the formation and evolution of the interfaces between steel and Al was analyzed. Two
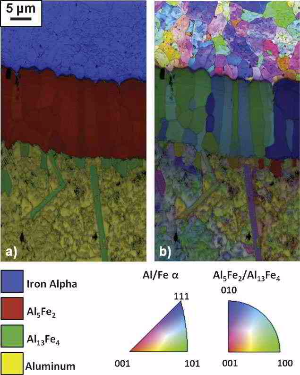
intermetallic compounds were found at the interface, namely, one adjacent to the steel layer (Al5Fe2) and one close to the solidified Al (Al13Fe4). The thickness of the intermetallic reaction
layer increases with laser power, while the morphology of its individual components evolves due to differences in accumulated thermal cycles. Correlations between simulations and
measurements show that the peak
temperature has significantly stronger influence on the thickness of the intermetallic reaction layer than cooling time and the integral of temperature over the time. Shear/tensile strength
tests reveal that all the specimens fail in the Al
heat affected zone.
Acta Materialia 96 (2015) 203-211
H. Springer, A. Szczepaniak, D. Raabe
On the role of zinc on the formation and growth of intermetallic phases during interdiffusion between steel and aluminium alloys
Acta Materialia 96 (2015) 203 Zn effect [...]
PDF-Dokument [980.2 KB]
Dissimilar joining between iron (Fe) based steels and aluminium (Al) alloys is the key to blend the attractive property profiles of these most common structural materials in one hybrid part.
Steels are mainly chosen for contributing cost effective strength and high stiffness, Al alloys provide low density, high heat conductivity and excellent corrosion resistance [1]. Examples for such
hybrid designs are lightweight body-in-white structures in transportation systems [2,3], protective Al coatings on steel sheets or tubes [4,5], or heat exchangers in energy conversion systems [6,7].
In all those applications an intimate bond between both materials is required, thus necessitating the application of thermal
joining procedures opposed to mechanical fasteners such as screws or rivets [8]. Adhesive bondings are typically not only less cost effective, but also require overlapping flanges (which increase
weight), are limited in the maximum application temperature and deteriorate the thermal and electrical conductivity [9]. The greatest challenge in the application of thermal joining procedures for
the dissimilar joining of steel and Al alloys is the formation of inherently brittle intermetallic reaction layers at the interface. Depending on the reaction conditions determined by the respective
joining process (e.g. time/temperature cycle, surface conditions, deformation), the reaction layers are complex in terms of build-up and morphology [10–12]. The g phase (Al5Fe2) has been identified
as the most prominent component in such dissimilar joints, which is widely acknowledged to be caused by its rapid growth kinetics facilitated by the open and anisotropic crystallographic
arrangement [13,14]. This prevalence of the g phase is of great relevance for the dissimilar joining of Al alloys and steel as
it has been reported to be one of the most brittle intermetallic phases of the Al–Fe system [15,16]. Many studies have indicated the detrimental effect of growing reaction layers on the
mechanical properties and the critical thickness has been found to be in the range of 3 – 10 lm, with different microstructural mechanisms deteriorating the joint strength and ductility
[11,17,18]. Consequently, numerous process variations have been developed, both for solid-state joining (e.g. friction welding, diffusion bonding etc. [19–21]) and ‘solid/liquid’ techniques (e. g.
Arc- or Laser-processes, where the Al alloy is molten and wets the solid steel [22,23]), all aimed at minimising and controlling the intermetallic layer thickness and build-up to improve the joint
properties.
However, the chemical compositions of the reacting alloy systems – i.e. steel, Al alloy and, if used, filler material – have also a pronounced influence on the intermetallic phase formation
and growth. The well-known retarding effect of silicon (Si) additions to Al on the growth kinetics of reaction layers is for example long since exploited in Al dip-coatings on steel, even though
the mechanism of the growth suppression is still not completely understood [4,10,24,25]. Gebhardt and Obrowski [26] reported that additions of zinc (Zn), on the other hand, have the opposite effect
of Si, i.e. leading to rapidly accelerated reaction layer growth up to a factor of more than 50 compared to reactions with pure Al. The growth acceleration was not found to increase linearly with the
Zn content, but rather to exhibit a maximum at additions of 10 wt.% Zn [26]. Understanding this effect of Zn is not only important from an academic point of view, but also of enormous technological
interest, as Zn-coatings are the most common corrosion protective measures for steels, especially on thin sheet material used in the automotive industry, and thus a common component in the
reaction zone in dissimilar Al/steel joints [1]. Many different techniques have been developed to obtain such Zn-coatings, such as galvanic hot dip-coating (as the most commonly applied
process), electrolytic deposition, powder/vapour-processes or even organic coatings [27–29]. All of these processes lead to Zn-coatings with thickness values ranging from about five to several
hundred lm, and of specific microstructural and chemical characteristics related to the applied treatments. In dip-coating processes for example, small additions of Al (about 0.1 wt.%) are added to
the Zn bath, which lead to the formation of a very thin (nm range) layer of an Fe–Al intermetallic reaction layer on the steel substrate [27]. This ‘inhibition layer’ in between steel and
coating is reported to act as a diffusion barrier for Zn, thus suppressing rapid growth of Fe–Zn intermetallic phases (‘outbursts’) during galvanising, which have been found to strongly decrease the
bond quality [30]. Apart from being coincidentally present in the reaction zone stemming from a steel coating during welding, Zn may also be utilised as an alloying element to contribute its cathodic
effect for novel Al-based protective coatings on steel [29]. In light of the large variety of joining processes and respective parameters, base materials and types of coatings, it becomes clear that
systematic investigations of the underlying phenomena for the intermetallic phase formation and growth are of great interest in order to derive guidelines for the knowledge-based optimisation of
dissimilar joining and coating technology.
The objective of this work is to elucidate the role of Zn on the formation and growth of intermetallic reaction layers formed at the interface between steel and Al alloys at elevated
temperatures as present in thermal joining procedures. The reaction products are studied in controlled experiments concerning the reaction between pure as well as Zn-containing Al and low C
steel with and without different types of Zn-coatings in direct comparison, in experiments above and below the melting point of Al.
More specific here the effect of Zn – both within Al and as a coating on steel – on the intermetallic phase formation and growth was systematically studied in controlled experiments, simulating the
interfacial reactions taking place in dissimilar solid/solid and solid/liquid joining procedures. Independent from the reaction temperature, the addition of 1.05 at.% Zn (2.5 wt.%) to Al had no
effect on the reaction layers’ build-up with the η phase (Al5Fe2) as the dominant component, but accelerated their parabolic growth up to a factor of 13. While Zn-coatings on steel were found to be
beneficial for the regular and even formation of intermetallic reaction zones in solid/liquid joining procedures, their role in solid-state processes was found to be more complex and, if no
countermeasures are taken, extremely detrimental to the joint properties. Possible reasons for the Zn-induced growth acceleration are discussed, as well as consequences for possible optimisation
steps for reducing harmful effects of Zn in dissimilar joints between Al alloys and steel.
Materials Science and Engineering A 528 (2011) 2641-2647
J. Song, A. Kostka, M. Veehmayer, D. Raabe
Hierarchical microstructure of explosive joints: Example of titanium to steel cladding
Mater Sc Engin A 528 (2011) 2641 Ti stee[...]
PDF-Dokument [627.8 KB]
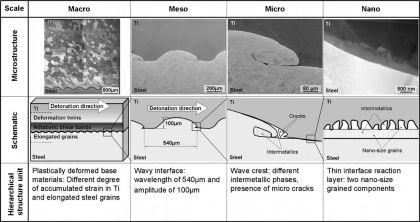
The microstructure of explosive cladding joints formed among parallel Ti and steel plates was examined by electron microscopy. The bonding interface and the bulk materials around it form
pronounced hierarchical microstructures. This hierarchy is characterized by the following features: at the mesoscopic scale
of the hierarchy a wavy course of the interface characterizes the interface zone. This microstructure level is formed by heavy plastic shear waves wavelength≈0.5mm) which expand within the two
metal plates during the explosion parallel to the bonding interface. At the micro-scale range, intermetallic inclusions (size≈100–200m) are formed just behind the wave crests on the steel
side as a result of partial melting. Electron diffraction revealed FeTi and metastable Fe9.64Ti0.36. Most of the observed phases do not appear in the equilibrium Fe–Ti phase diagram. These
intermetallic inclusions are often accompanied by micro-cracks of similar dimension. At the smallest hierarchy level we observe a reaction layer of about 100–300nm thickness consisting of
nano-sized grains formed along the entire bonding interface. Within that complex hierarchical micro- and nanostructure, the mesoscopic regime, more precisely the type and brittleness of the
intermetallic zones, seems to play the dominant role for the mechanical behavior of the entire compound.